News Story
NIH Awards to Fund Research for Tissue Chips

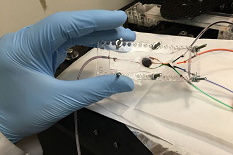
This lung-on-a-chip serves as an accurate model of human lungs to test for drug safety and efficacy. Wyss Institute for Biologically Inspired Engineering, Harvard University Photo
More than 60 percent of investigational drugs fail in human clinical trials due to a lack of effectiveness, despite promising pre-clinical studies using cell and animal research models. To help combat this translational science problem, the National Institutes of Health announced 13 two-year awards totaling about $15 million per year, with FY18 funds subject to availability, to develop 3-D microphysiological system platforms that model human disease. The funding is for the first phase of a five-year program. These platforms, called “tissue chips,” support living cells and human tissues to mimic the complex biological functions of human organs and systems and provide a new way to test potential drug efficacy.
These Tissue Chip for Disease Modeling and Efficacy Testing awards may enable scientists to better understand disease mechanisms and predict more accurately how patients will respond to specific drugs. The support is made possible through NIH’s National Center for Advancing Translational Sciences' (NCATS) Tissue Chip for Drug Screening program, which leads this effort in collaboration with other NIH Institutes and Centers.
“The goal is for these tissue chips to provide more accurate platforms to understand diseases, and to be more predictive of the human response to drugs than current research models, thereby improving the success rate of candidate drugs in human clinical trials,” said NCATS Director Christopher P. Austin, M.D.
NCATS launched its Tissue Chip program in 2012 to lead the development of highly innovative microphysiological systems to study drug safety and toxicity in a faster, more effective way than current methods. The tissue chips can be integrated to form a human body-on-a-chip, enabling researchers to study investigational drugs and therapeutic agents across the entire body prior to human clinical trials.
The new Tissue Chip awardees will study a wide range of common and rare diseases, from rheumatoid arthritis, kidney disease and human influenza A viral infection to amyotrophic lateral sclerosis (ALS), hereditary hemorrhagic telangiectasia and arrhythmogenic cardiomyopathy.
In the second phase of the awards, researchers will partner with pharmaceutical companies to further evaluate the usefulness of validated disease models – those that accurately mimic disease biology – in assessing the effectiveness of candidate drugs.
In addition to NCATS, NIH Institutes and Centers funding the new awards include the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institute of Biomedical Imaging and Bioengineering, National Institute for Diabetes and Digestive and Kidney Diseases, National Institute of Dental and Craniofacial Research, National Institute of Environmental Health Sciences, National Heart, Lung and Blood Institute, National Institute of Neurological Disorders and Stroke, Eunice Kennedy ShriverNational Institute of Child Health and Human Development, and the NIH Office of Research on Women’s Health.
The 2017 awardees are:
Brigham and Women’s Hospital, Boston
Joseph Vincent Bonventre, M.D., Ph.D., and Luke Lee, Ph.D. (University of California, Berkeley)
Kidney Microphysiological Analysis Platforms to Optimize Function and Model Disease
Grant Number: 1-UG3-TR-002155-01
Cedars-Sinai Medical Center, Los Angeles
Clive Niels Svendsen, Ph.D.
Development of a Microphysiological Organ-on-Chip System to Model ALS and Parkinson’s Disease
Grant Number: 1-UG3-NS-105703-01
Columbia University, New York City
Gordana Vunjak-Novakovic, Ph.D.
Multi-Tissue Platform for Modeling Systemic Pathologies
Grant Number: 1-UG3-EB-025765-01
Duke University, Durham, North Carolina
George A. Truskey, Ph.D.
Systemic Inflammation in Microphysiological Models of Muscle and Vascular Disease
Grant Number: 1-UG3-TR-002142-01
Harvard University, Cambridge, Massachusetts
Donald E. Ingber, M.D., Ph.D.
Lung-on-a-Chip Disease Models for Efficacy Testing
Grant Number: 1-UG3-HL-141797-01
Harvard University, Cambridge, Massachusetts
Kevin Kit Parker, Ph.D., and William Tswenching Pu, M.D.
Multi-Scale Modeling of Inherited Pediatric Cardiomyopathies
Grant Number: 1-UG3-HL-141798-01
Northwestern University, Evanston, Illinois
Teresa K. Woodruff, Ph.D.
Polycystic Ovary Syndrome and Androgen-Related Disease Modeling and Drug Testing in Multi-Organ Integrated Microfluidic Reproductive Platform
Grant Number: 1-UG3-ES-029073-01
University of California, Davis
Steven Carl George, M.D., Ph.D., David Terry Curiel, M.D., Ph.D., (Washington University in St. Louis) and Stacey Lynn Rentschler, M.D., Ph.D. (Washington University in St. Louis)
A 3-D In Vitro Disease Model of Atrial Conduction
Grant Number: 1-UG3-HL-141800-01
University of California, Irvine
Christopher C.W. Hughes, Ph.D.
Microphysiological Systems to Model Vascular Malformations
Grant Number: 1-UG3-HL-141799-01
University of Pittsburgh
Rocky S. Tuan, Ph.D.
Tissue Chip Modeling of Synovial Joint Pathologies: Effects of Inflammation and Adipose-Mediated Diabetic Complications
Grant Number: 1-UG3-TR-002136-01
University of Rochester, Rochester, New York
Danielle S. Benoit, Ph.D., Lisa A. Delouise, Ph.D., and Catherine Ovitt, Ph.D
Engineered Salivary Gland Tissue Chips
Grant Number: 1-UG3-DE-027695-01
University of Washington, Seattle
Jonathan Himmelfarb, M.D.
A Microphysiological System for Kidney Disease Modeling and Drug Efficacy Testing
Grant Number: 1-UG3-TR-002158-01
Vanderbilt University, Nashville, Tennessee
Aaron B. Bowman, Ph.D., Kevin C. Ess, M.D., Ph.D., and John Peter Wikswo, Ph.D.
Drug Development for Tuberous Sclerosis Complex and Other Pediatric Epileptogenic Diseases Using Neurovascular and Cardiac Microphysiological Models
Grant Number: 1-UG3-TR-002097-01
Read more at: https://www.nih.gov/news-events/news-releases/nih-awards-15-million-support-development-3-d-human-tissue-models
Published November 7, 2017